Soil
Nutrients in the soil
Nutrients refers those elements required by plants to grow and survive. Macro nutrients are ones that are needed in larger quantities, called major elements, such as nitrogen (N), phosphorus (P), and potassium. These three are grouped together as NPK. Micro nutrients are ones required in smaller quantities, called trace elements and includes iron (F), boron (B), manganese (Mn), magnesium (Mg) and molybdenum (Mo). Each one has an important role to play in the growth of plants.
There are a number of variants which will determine the availability of these nutrients. The two major factors involved are the pH of the soil and the ability of the soil to hold nutrients which is called its cation exchange capacity (CEC).



Soil pH
The pH levels in the soil have a direct effect on the availability of nutrients to plants.
The technical stuff
pH measures the concentration of hydrogen ions. This has a direct correlation to cation exchange (see below) in the soil. Hydrogen ions are positively charged and will attach to the negative sites (colloids) in the soil particles.
- At higher pH there is a low concentration of hydrogen ions so there are more sites available for other elements to attach; reducing their likelihood of leaching out and allowing them to be held in the soil for plants to access.
- At lower pH there is a higher concentration of hydrogen ions, preventing other elements from attaching to the sites. These other elements are then easily leached out and less likely to be taken up by the plants.
Elements also bind to the soil in varying degrees at different pH levels.
- At high pH metal elements such as iron (Fe), zinc (Zn), manganese (Mn), copper (Cu) and aluminium (Al), are tightly bound to the soil and not readily available to plants. At low pH they are more available and at high concentrations can become toxic to plants
- At low pH ‘base’ elements such as calcium (Ca), potassium (K) and Magnesium (Mg) are weakly bound and thus prone to leaching. Molybdenum (Mo), phosphorus (P), magnesium (Mg), and calcium (Ca) are less available.
- At high pH calcium ties up phosphorus making it less available.
Soil microbiology is greater around the neutral (pH 7) level.
The pH of a soil will tell us if the soil is acidic (pH lower than 7) or alkaline (pH higher than 7). Different ranges of pH affect the availability of nutrients. Each pH level is 10 times that of the previous one e.g. pH 6 is ten times more acidic than pH 7 and pH 5 is one hundred times more acidic than pH 7. A seemingly small pH difference of 0.5 can therefore have a large impact on the growth of plants, depending on what you’re trying to grow.
Most nutrients are available to plants in the range pH 5-7.5. Outside of this some nutrients become ‘locked up’ and are unable to be taken up by the plant. If you suspect that one of your crops has a nutrient deficiency, it may be that the pH is out and adding more nutrients will not solve the problem.


Nutrient availability
Each crop will experience optimum growth in a certain pH range. Blueberries grow best at around pH 5, potatoes at around pH 6, and beetroot at around pH 7. A detailed list of pH preferences can be found in the pH Requirements of Crops entry in the planting guide category.
The good news is that under organic growing conditions the availability of nutrients covers a greater pH range than with conventional growing practices.
Deficiency vs toxicity
A deficiency is when plants are not getting as much of a nutrient or mineral as they need, and toxicity is when the concentration is too high and they are effectively getting too much of a good thing.
Nutrient deficiency or toxicity is very difficult to diagnose by non-experts as the symptoms, such as discolouration of plant leaves, are not always caused by nutrient imbalances. Also different nutrient deficiencies show as subtle variations in the way the leaves yellow. Yellowing of the leaves could be caused by a nitrogen deficiency, a sulphur deficiency, too much water or not enough. Purple colouring of the leaves can be caused by a phosphorus deficiency or the accumulation of certain carbohydrates.
A high concentration of a particular mineral may result in deficiencies of other essential minerals.
- High calcium can cause deficiencies of potassium and magnesium.
- Excess zinc or copper can result in an iron deficiency.
- If there is a low level of molybdenum, nitrogen isn’t able to be utilized and plants will show symptoms of nitrogen deficiency, such as yellowing of their leaves
In this instance there may already enough of a nutrient in the soil but it is unable to be taken up by the plant. Adding more will not help
The only sure way of knowing is to have a soil test done.
If the leaves are yellowing or the plant looks sick, check the pH first before adding nutrients. Small test kits are available from gardening supply retailers.
Ensuring that your soil is healthy with good compost and fertilizers and healthy seedlings is a good start. Your plants will soon tell you if there is a problem.
The chart shows the availability of each nutrient element at the various pH levels. The widest parts of the bands are where there is the maximum nutrient availability.
NB: The range at which the nutrients are most available is 6.5 to 7.5 which is slightly alkaline, neutral and slightly acidic.
Phosphorus reacts with other elements at pH levels that are higher (alkaline, over 8.0) and lower (acidic, below 5.0) in a way that makes it less available to plants.
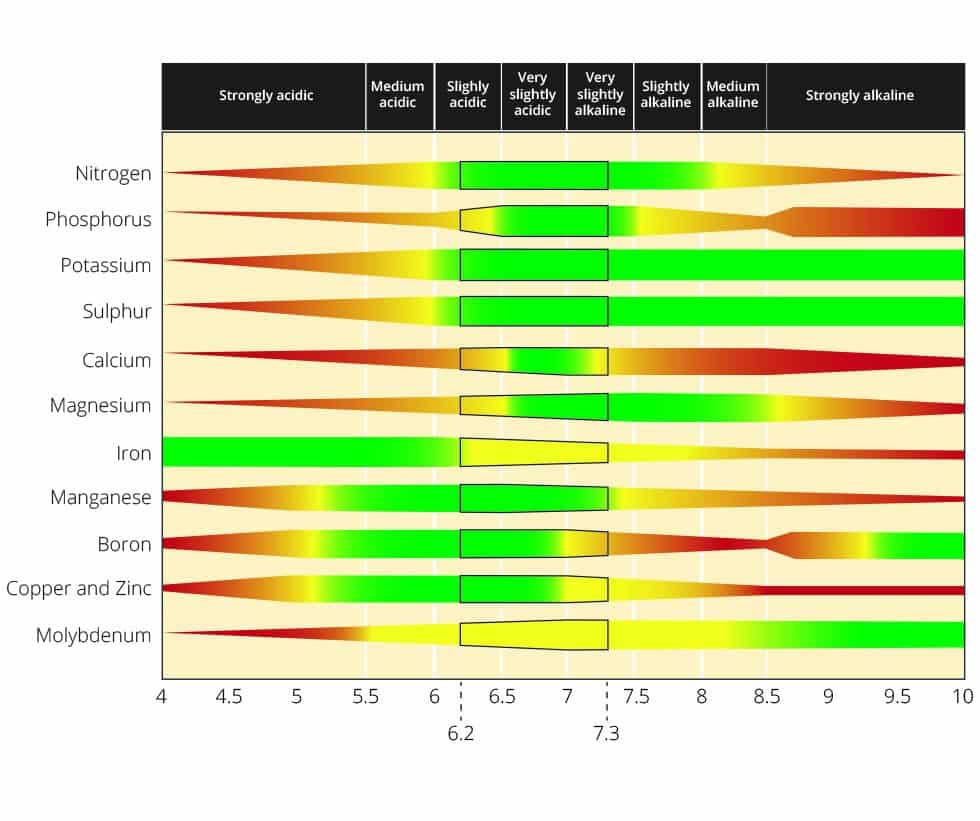
The widest parts of the bands are where there is the maximum nutrient availability.
cation exchange capacity (CEC)
It’s also important that the soil be able to hold on to and store nutrients. Even if nutrients are present in an available form, the soil will not be productive for long if these nutrients leach out of it quickly. In organic gardening we measure how well the soil can hold nutrients by its “cation exchange capacity”. Cations are the ions of elements such as calcium, magnesium, potassium, sodium, and aluminium that have a positive charge.
The particles of clay and humus that hold the cations are negatively charged and are called colloids. The particles are thin and flat with a large surface area for the cations to attach to. The cations (positively charged) are attracted to, and held by the colloids (negatively charged). They are, in effect, a “warehouse” for many of the minerals and nutrients required by our plants. As plants take up nutrients from the colloids more nutrients replace them. When we speak of soils that are “colloidal”, we’re saying that they have a high cation exchange capacity and should remain productive over time if we look after them.
There are a number of factors that influence how well and which particular nutrients are held by the colloids. It is important to keep in mind that a high concentration of a particular nutrient will effectively “saturate” the colloids and make it difficult for the less concentrated cations to bond and be held by the colloids.
The pH of soil also has a complex influence on its ability to hold on to nutrients. An acidic soil (low pH) favours metal cations such as iron, aluminium and copper. Conversely, the high pH range favours the carbonates such as boron, phosphorus and molybdenum. The negative charge on colloids increases with pH, providing maximum availability at around pH 7-7.5, but extremes at either end of the pH range can be detrimental; for example, at very high pH values carbonate cations will reach toxic levels and prevent other major and trace elements from being held by the colloids.
Fortunately a healthy soil with a good level of organic matter will look after all this for us. If you get your soil professionally tested and are told it has a low cation exchange capacity, it can always be improved by adding organic matter.
Humus, the end product of decomposition, has the highest capacity for holding nutrients.
Worm castings are also extremely colloidal, more than clay.
Clay has a very high capacity for holding nutrients, although this does vary with the different types of clay.
See the information in the Product inputs entry in the Soils section for additional ways of increasing organic matter in the soil.






Boron
What does it do?
- helps to form cell walls as the plant grows
- aids in pollen germination and flowering
- boosts the uptake of silicon by making it soluble
Boron deficiency
- reduces the uptake and use of calcium by the plant
Results in:
- abnormal growth
- thick, curled leaves
- poor root development
- poor fruit set
- misshapen and cracked fruit
- growing tip die-off.
Causes of boron deficiency
- boron is a negatively charged element and therefore won’t be attracted to the colloids (see cation exchange capacity above) and therefore is not stored in the soil
- it is soluble and can be leached out easily
- common in sandy soils
- high rainfall areas cause it to be leached out easily
- high soil pH
- high calcium concentration; important not to use a calcium product which will increase the pH of the soil if it is already alkaline
- drought
- soil low in organic matter
To correct
- add humus to sandy soils
- keep pH at a lower level
- do not add too much lime (calcium)
- seaweed foliar spray
- add more organic matter- boron is released as it decomposes
Toxicity
Boron reaching toxic levels is generally not common in natural circumstances as it tends to leach out of the soil easily.
If it were a problem it would cause tips of older leaves to yellow then fall off.
As it is only required at a very low level, toxicity could occur when it is being intentionally added to the soil
Application:
Commercial products will state application rates and methods.
Comment:
For a quick fix, borax can be used to add boron to soil.
We would apply borax if our soil test indicated that boron was required or if we saw symptoms of a deficiency in our plants. Mix 1 tablespoon in 12 litres of water, dissolving the borax in a little hot water first. You do need to be careful because it is very easy to overdo it and reach a toxic level.
Plants requiring higher levels of boron are apples, cabbage family, strawberries, celery, lettuce, spinach, beetroot, turnips, and sunflowers.
Boron is more likely to be deficient in soils formed from granite or sandstone as opposed to those soils developed from basalt or alluvial origins or those with a high content of clay.

Calcium
What does it do?
- aids - cell division (especially important where rapid growth occurs, as with tomatoes)
- disease resistance
- pollen germination
- increases pH of the soil if added in a lime product
- improves biological activity by helping to maintain a good environment for the microbes in the soil. Byproducts from microbial activity are alkaline
- helps to keep chemical balance in the soil
Calcium forces fine clay particles to hold together in larger groups, resulting in larger peds (permanent aggregates of soil) with more space between them which increases aeration, allows water in and good drainage out, creates more room for roots within the soil.
Deficiency
- most obviously as blossom end rot on fruits.
- young growing tips not developing properly and die off
- leaf necrosis (injury or death of cells)
- short brown roots, fungal issues
- weak stems
- stunted growth
- damping off
- reduced disease resistance
- the leaf tip and edges yellow and the stems are weak.
Causes of calcium deficiency
- acidic soils tend to have less calcium
- calcium is taken up with water so if there is a problem with the plant taking water, there will be less calcium getting to the plant
To correct
Add calcium in one of the following forms:
Agricultural Lime (most commonly used)- contains 35% to 38% calcium with very little magnesium
- raises the soil pH (i.e. makes it more alkaline)
- not desirable to use it around plants that like an acidic soil
Gypsum (calcium sulfate) -contains calcium and sulphur -is pH neutral so it won't change the soil pH -it is more soluble than other limes -will add sulphur
Limestone (pure calcium carbonate)- contains 40% calcium and no magnesium
- raises the soil pH (i.e. makes it more alkaline)
- finely ground calcite limestone acts as a slow release form
Dolomite (calcium magnesium carbonate)- contains 12% to 20% calcium and 8% to 11% magnesium
- raises the soil pH (i.e. makes it more alkaline)
- is an untreated limestone rock that also contains magnesium
Soft rock phosphate - calcium and phosphorus
- very slow to break down
- needs to be dug into the soil to help it dissolve
Eggshells - use as a ‘home grown’ additive
- very slow releasing
- need to grind up finely and dig into soil
Oyster shell - Crushed shells are usually oyster and other shellfish and although they are almost totally calcium carbonate they are very hard and coarse to be effective.
Calcium toxicity
- not generally considered toxic
- if too much lime has been added the soil pH may be raised too much, making other nutrients less available.
Application
- autumn is a good time to add calcium when preparing new beds for spring
Comments
Adding lime and/ or eggshells to compost is a good way to increase soil calcium
The lime needs to be fine (in powder form) to react quickly with the soil.

Copper
What does it do?
- is an important component of enzymes, mainly in plant roots
- aids plant metabolism
Deficiency results in:
- stunted growth
- die back of shoot tips in trees and shrubs
- distorted leaves (sometimes bleached or curled)
- pale flowers, particularly in fruit trees
Causes of copper deficiency
- high levels of iron, magnesium, zinc or phosphorus
- leached sandy soil
- too much lime, causing high pH
To correct
- apply copper chelate as foliar spray
Copper toxicity
- can occur if copper sprays are used regularly
Application
- only if a soil test indicates it is lacking

Iron
What does it do?
- regulates and promotes plant growth
- essential for producing chlorophyll and for the process of photosynthesis
- helps plant respiration.
Deficiency
- causes the area between leaf veins to turn pale green then yellow, while the veins remaining green.
It starts in the young leaves, progressing to the older leaves and eventually the whole leaf bleaches. As a result the plant is unable to photosynthesize.
Causes of iron deficiency
- often occurs in plants that prefer acid soils but are being grown in alkaline soil
- alkaline soil making iron unavailable to the plant
- too much lime or over-application of potassium, copper or zinc
- soil high in molybdenum or phosphorus will reduce the uptake of iron
To correct
- lower the pH if it is high by adding manures, compost, mulch and leaf litter -these are generally acidic
- reduce use of fertilizers with lime and phosphate in them
- use products which are very low in phosphorus such as blood meal or pine bark mulch
- plant nitrogen-fixing plants such as legumes- these tend to lower the pH of the soil
- for a quick fix, foliar spray with iron chelate or iron sulfate- reacts in 3-4 weeks
Iron toxicity
- not common but occurs more often in wet(flooded) conditions
- will show up as dark brown spots on older leaves
Comments
- iron occurs naturally in soils but becomes less available to the plants at high pH

Magnesium
What does it do?
- important component of chlorophyll and is necessary for photosynthesis
- activates enzymes
- important for plant respiration
- carries phosphorus through the plant
Deficiency
- causes patchy yellowing between the veins of the leaves, starting at the tips and spreading toward the stem. It will start in the older, lower leaves, before spreading to the younger leaves
- lower yields
- increases susceptibility to diseases
Causes of magnesium deficiency
- less available in acidic soils
- heavy use of potassium
- sandy acid soils in high rainfall areas are usually deficient as it is easily leached out
To correct
- use dolomite (magnesium calcium carbonate)
- magnesite (magnesium carbonate)
- Epsom salts (magnesium sulphate)
- seaweed products
Magnesium toxicity
- not common but may occur if using bore water that is high in sodium.
- will result in reduced growth rates

Manganese
What does it do?
- helps with photosynthesis
- helps with respiration
- metabolizes nitrogen
Manganese deficiency
- shows up first in young leaves as yellowing between the veins; may also be visible as brown spotting near the primary leaf veins
- more likely in sandy soils.
Causes of manganese deficiency
- when pH is raised too high
- extended periods of cold, overcast weather which may limit photosynthesis
To correct
- apply manganese sulfate as a foliar spray
- use a complete fertilizer containing all the trace elements
Toxicity
- causes older leaves to get dark spots with a yellowing rim
- more common in very acidic soils with a pH of 5.5 or lower
Comments
- adding lime will fix toxicity

Molybdenum
What does it do?
- helps bacteria and soil organisms convert atmospheric nitrogen to a form that is available to plants
- particularly needed by legumes
Molybdenum deficiency
- will stop nitrogen from being used, thus the visible symptoms are those of nitrogen deficiency; yellow older leaves, reduced growth, and long, thin leaves
- molybdenum is a negatively charged element and therefore won’t be attracted to colloids (see cation exchange capacity) and won’t be stored in the soil
Causes of molybdenum deficiency
- usually occurs in acidic and sandy soils
To correct
- maintain a higher pH
- use a fertilizer with trace elements
- spray or drench using seaweed products
- compost
- mulch
- plant green manure crops
Toxicity
- rare
Comments
- Molybdenum is usually contained in the product used to inoculate legume seeds used for green manure crops.

Nitrogen
What does it do?
- found in all plant cells, proteins, hormones and chlorophyll
- promotes leaf growth
Nitrogen deficiency
- turns oldest leaves pale green to yellow, beginning at the leaf tips then spreading to the whole plant
- growth is stunted
Causes of nitrogen deficiency
- nitrogen is easily leached out of the soil by rain, so small amount need to be applied often
To correct
- add fish emulsion
- composted animal manures (particularly chicken) as solid or liquid fertilizer
- compost
- blood and bone
- use green manure crops
- legumes will ‘fix’ atmospheric nitrogen in the soil (bacteria are present in their root nodules)
- inoculation (coating the seed with specific nitrogen ‘fixing’ bacteria) of green manure crops such as legumes, wheat and lentils.
Iron toxicity
- occurs when high rates of nitrogen fertilizers are applied
- will result in excessive leaf growth and poor fruit growth
Comments
- most of the nitrogen in soils is produced by legumes and soil organisms
- composted manures will reduce leaching
- soil high in organic matter is generally higher in nitrogen
- leafy crops such as lettuce, silver beet, and kale require lots of nitrogen
- Using legume crops in rotation or as green manure between crops is the most efficient and effective way of securing nitrogen in the soil.

Phosphorus
What does it do?
- aids photosynthesis
- stimulates early root development
- promotes flower, fruit and plant growth
- hastens maturity
Phosphorus deficiency
- causes leaves to turn a reddish-purple (oldest leaves first)
- growth is stunted.
Causes of phosphorus deficiency
- it is not soluble so won’t leach out of the soil, but tends to bind to soil particles
- many Australian soils have low levels of phosphorus as they are “phosphorus fixing”; the phosphorus is tightly bound to the soil (particularly acid soils) and relatively unavailable to the plants
- soil is too acidic or too alkaline
- phosphorus is a negatively charged element and therefore won’t be attracted to colloids (see cation exchange capacity) and won’t be stored on them
To correct
- pH level of 6 to 7.5 for optimum phosphorus uptake
- biochar
- blood and bone
- fish emulsion
- rock phosphates; especially good added to the compost where the composting action helps release the phosphorus in an organic form.
- sulphate of potash
- seaweed foliar spray
- chicken manure; all manures contain phosphorus (poultry and grain fed animals are a rich source), in a form that is not fixed by the soil. In the long term they can reduce phosphorus fixation.
Phosphorus toxicity
- excess phosphorus caused by over application of phosphorus fertilizer will result in zinc and/or iron deficiencies
- the plants’ ability to take up zinc, iron and other micro nutrients is reduced.
- usually as a result of inorganic fertilizers, or of composts and manures that are excessively high in phophorus.
Comments
- Certain fungi can help plants to extract phosphorus. VAM (vesicular-arbuscular mycorrhizae) fungi can be encouraged by minimizing tillage, using winter cover crops, and crop rotation. VAM is often used to inoculate (coat the seeds) of green manure crops prior to planting.
Peas particularly require phosphorus.

Potassium
What does it do?
- it is the third major requirement of plants after nitrogen and phosphorus
- particularly aids plants in their flowering and fruiting
- increases vigour
- increases disease resistance
- helps form and transport starches, sugars and oils in plants
- strengthens the cell walls which in turn gives the plants protection against frost
- can improve fruit quality by improving yield, nutrient content, taste, colour and texture
- improves water retention
Potassium deficiency
- causes the edges of the leaves to turn grey or tan, affecting the oldest leaves first
- puckering of leaf edges
- fruit poor quality
Causes of potassium deficiency
- common in sandy soils
To correct
- potash (wood ash), but this will also raise the pH
- sulphate of potash
- seaweed fertilizer or kelp meal
- comfrey spray
- compost made with a lot of fruit and vegetable waste, especially banana peels, citrus rinds, spinach and tomatoes
- composted animal manures
Potassium toxicity
- excess potassium caused by over fertilizing will result in a magnesium deficiency
Silicon
I think that silicon is a bit underrated, as very few gardeners ever talk about it, but as you can see, it has many fine attributes.
What does it do?
- strengthens cell walls, which can increase the plant’s stress resistance
- improves drought resistance, salt tolerance and soil fertility
- reduces insect attack and susceptibility to disease
- minimizes frost damage
- helps retain water
- neutralizes heavy metal toxicity
- counters the effects of excess sodium
- increases root growth
- optimizes the uptake of nutrients, thus improving the quantity and quality of crops
Silicon deficiency
- most plants will grow well without silicon as it not an essential nutrient.
Comments
- Clay has silicates and sand is mostly silicon but not in a form that is available to plants.
- Diatomaceous earth contains 85% silicon and is sold in powdered form that can also be applied in water.
- Mineral dusts (green sand)contain good quantities of silica; palagonite a basalt dust, is an extremely good source.
Sulphur
What does it do?
- present in amino acids in plant proteins
- helps in the formation of chlorophyll
- responsible for the flavour and aroma of plants such as onion and cabbage
- fights fungus
- legumes need sulphur to ‘fix’ nitrogen
Sulphur deficiency
- causes overall yellowing of leaves and stunted growth, showing up first in the new growth
- common in soils with low organic matter, very sandy soils with high rainfall, and in waterlogged soils
Causes of sulphur deficiency
- sulphur is water soluble and leaches out of the soil easily
- it is a negatively charged ion and therefore will not be able to attach to colloids (refer to cation exchange capacity) and won’t be held in storage
To correct
- high levels of organic matter will keep levels up
- gypsum will add sulphate.
- if pH is above 6, add a sulphate salt like Epsom salts as a drench
- add organic humus (compost will do)
- sulphate of potash
Sulphur toxicity
- not generally considered a problem as excess sulphur usually lowers the pH and sulphur uptake is reduced as soil pH decreases
- can cause salt burn on the edges of the leaf; leaves may drop off.
Comments
- Sulphides are common in soil but they must be converted into sulphates for plants to take up. There is a naturally occurring bacterium which will do this.

Zinc
What does it do?
- important in the production of a hormone responsible for stem and leaf growth
Zinc deficiency
- causes yellow mottling between the veins of young leaves, giving them a striped appearance
- causes rolling of the edges of leaves, which then die back and fall off
- plants have smaller leaves than they would normally
Causes of zinc deficiency
- high applications of lime (zinc becomes unavailable when pH is high)
- over use of iron or phosphate fertilizers
- common in red soils as zinc will combine readily with iron
To correct
- reduce use of phosphate and iron fertilizers
- apply zinc sulfate or zinc chelate as a foliar spray
- apply zinc minerals
Zinc toxicity
- results in iron deficiency.

